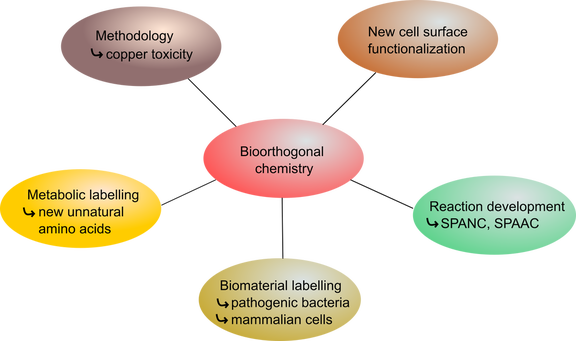
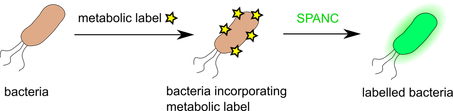
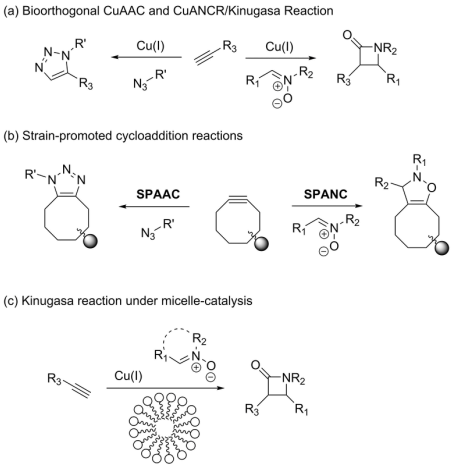
Our research group explores the field of bioorthogonal chemistry in several significant ways. Focus has been placed on developing and applying novel chemistry for bioorthogonal labelling of different biomaterials. To this end, variations of cycloaddition chemistry have been employed, derived from copper-catalyzed alkyne-azide cycloaddition (CuAAC). This chemistry has been expanded to the study of copper-catalyzed alkyne-nitrone cycloaddition reaction (CuANCR), as well as strain-promoted alkyne-azide cycloaddition (SPAAC). Furthermore, our group reported the development of reactions involving strain-promoted alkyne-nitrone cycloaddition reaction (SPANC). These reactions are among the fastest and most efficient reactions known and are highly effective in applications to bioconjugation in living systems. In addition to being highly used methods, we have now expanded the approach to the incorporation of nitrones into metabolic labels and used this method for detection of pathogenic bacteria.
|
Furthermore, our group was also able to establish important parameters by which to evaluate and design copper catalysts for applications in living systems. As well as, studying the role of copper and its coordination environment on its ability to catalyze bioorthogonal click reactions and the sources of toxicity of some copper species. We have contributed to the way in which the community thinks about both the design and utilization of copper species as catalysts in living systems.
|