Catalysis
Research in the area of catalysis follows two related tracks in our group: 1) mechanistic study and 2) applying catalysis to solve synthetic challenges that otherwise are difficult, if not impossible.
1) Mechanistic studies in catalysis:
While most catalytic reactions have mechanisms drawn out in textbooks, we learn new things about most reactions every day – one thing is for sure, no reaction is a simply as it may seem on the surface. Our group has been interested in the role of additives and how they impact catalytic reactions. In short, while not a starting material in the Negishi reaction, salt additives play a pivotal role that went unnoticed and/or poorly understood for decades. For arylzinc cross-coupling partners, we have proposed that simply table salt is necessary to both adjust the polarity of the cross-coupling solution to breakdown aggregates to make the organozinc more likely to undergo transmetallation. With alkylzincs, we propose (in blue in Scheme 1) that a different role for the salt exists leading to the formation of a more nucleophilic zincate (RZnX3)-2 and that that is the transmallating species and not the simple organometallic (in red) or the lower zincate (RZnX2)-1 as has been proposed by others.1
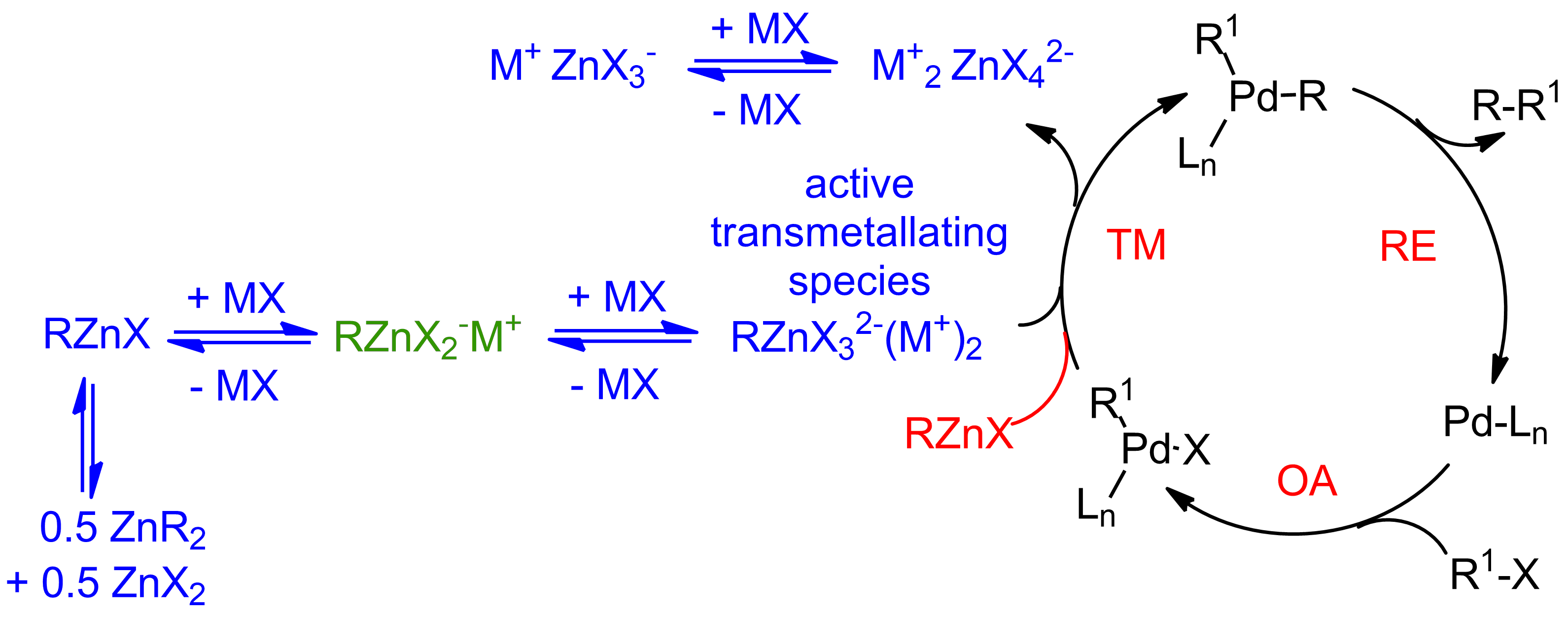
Scheme 1. Our proposed mechanism of the Negishi Reaction
We look at the mechanism of a number of cross coupling reactions (e.g., Suzuki Miyaura, Negishi, and cross coupling with nitrogen and sulfur nucleophiles) using a breadth of tools including kinetics, mass spectrometry, NMR spectroscopy, and calculations (in collaboration).
2) Solving old problems with new catalysts:
The importance of cross-coupling to organic synthesis cannot be overstated as evidenced by the awarding of the 2010 Nobel Prize to Negishi, Suzuki, and Heck. Earlier studies focused primarily on the coupling to two different aryl rings together – itself a major feat at the time and still the best and most general strategy to assemble to aromatic rings together. In addition to the formation of C-C bonds, cross-coupling can now be used essentially to make any linkage (e.g., C-N, C-O, C-S). Once it became clear that transition metals could be used to assemble electrophiles and nucleophiles, the bar has been raised to try to do such couplings with selectivity. For example, primary amines (Scheme 2a)2 or ammonia (Scheme 2b)3 can couple to one aryl electrophile, or it can over couple to generate the diaryl product.
a)
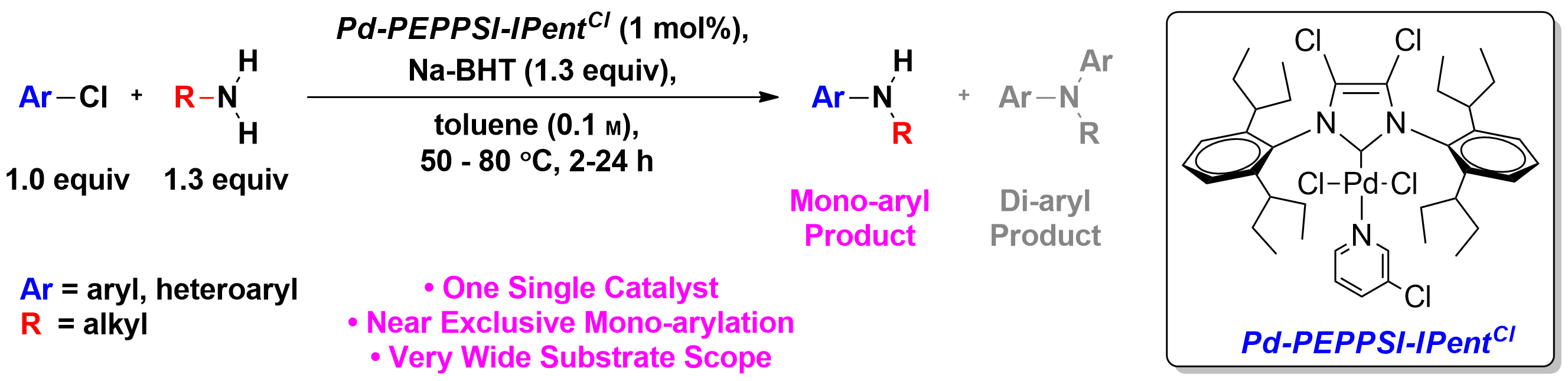
b)
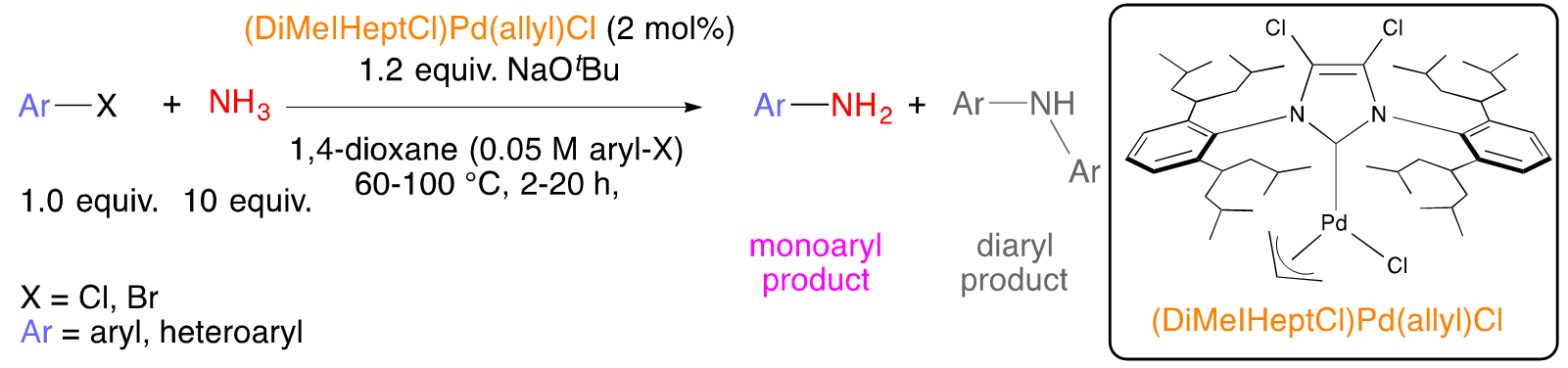
Scheme 2. Selective (hetero)arylation of a) primary amines and b) ammonia
Our group has also been highly active in the cross coupling of secondary nucleophiles to (hetero)arylhalides to produce tertiary centres adjacent to the ring without any migratory insertion that leads to the productions of isomers (Scheme 3).4
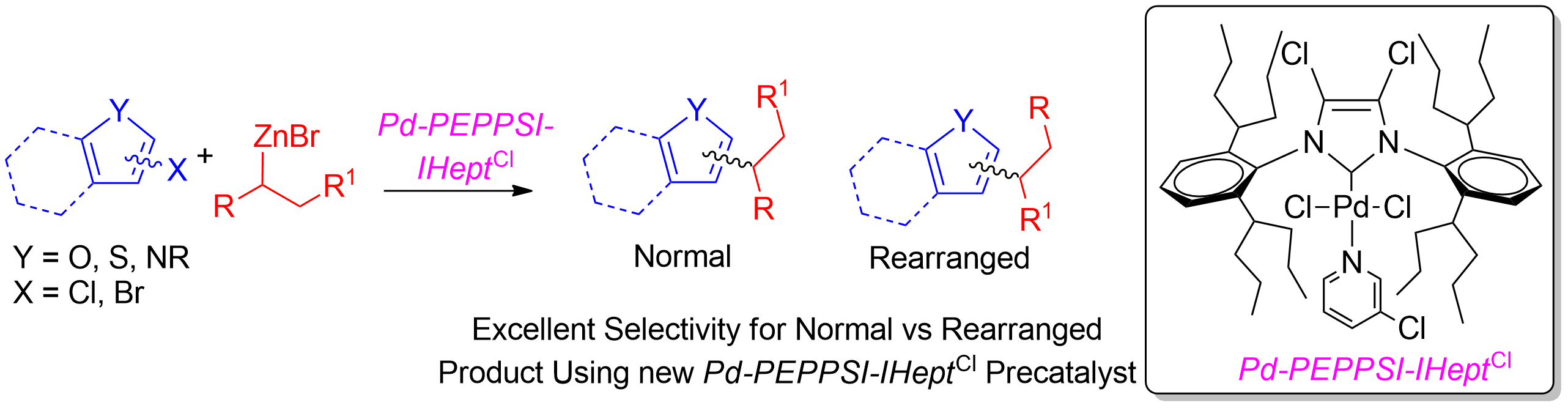
Scheme 3. Selective coupling of secondary alkylzinc nucleophiles to (hetero)aryl halides.
We collaborate with a large number of companies and our students and post-docs have spent time in the labs of the industrial partner.
Representative Publications (see full list of publication for all manuscripts):
1. McCann, L. A.; Organ, M. G. Angew. Chem. Int. Ed. 2014, 53, 4386-4389; McCann, L. C,; Hunter, H. N.; Clyburne, J. A. C.; Organ, M. G. Angew. Chem. Int. Ed. 2012, 51, 7024-7027; Hunter, H. N.; Hadei, N.; Blagojevic, V.; Patschinski, P.; Achonduh, G. T.; Avola, S.; Bohme, D. K.; Organ, M. G. Chem. Eur. J. 2011, 17, 7845-7851.
2. Sharif, S.; Rucker; R. P.; Chandrasoma, N.; Mitchell, D.; Rodriguez, M. J.; Pompeo, M.; Froese, R. D. J.; Organ, M. G. Angew. Chem. Int. Ed. 2015, 54, 9507-9511.
3. Lombardi, C.; Day, J.; Chandrasoma, N.; Mitchell, D.; Rodriguez, M. J.; Farmer, J. L.; Organ, M. G. Organometallics 2016 (Submitted).
4. Atwater, B.; Chandrasoma, N.; Mitchell, D.; Rodriguez, M. J.; Organ, M. G. Chem. Eur. J. 2016, 22, 14531-4; Atwater, B.; Chandrasoma, N.; Mitchell, D.; Rodriguez, M. J.; Pompeo, M.; Froese, R. D. J.; Organ, M. G. Angew. Chem. Int. Ed. 2015, 54, 9502 –9506.