Synthetic Chemistry In Flow
The synthesis of organic molecules has been conducted traditionally in a container such as a round-bottom flask on the lab scale, or in a large, often metal (e.g., stainless steel or hastelloy) stir-tank reactor on the manufacturing scale. This is referred to as ‘batch chemistry’. The production of large-quantity commodity chemicals, such as polymers and ammonia, are prepared in a different format, i.e., by pumping the reaction mixture continuously through a tube – this is called ‘flow chemistry’. Flow chemistry is a very effective way of synthesizing molecules as only small amounts of reactants are in the rube at any one time. This allows for greater control over mass and heat transfer in the reactor that leads to better chemical process, which leads to more efficient and cleaner transformations making flow both a ‘green’ technology and much safer owing to the small amounts of material undergoing reaction at any point in time. We are working at adapting flow chemistry to the preparation of organic molecules with the goal of realizing these same benefits in the preparation of high-value compounds, such as pharmaceutical agents.
Research in the group focuses on effective ways to utilize flow chemistry to realize benefits over the traditional batch processes. The group has pioneered the use of microwave irradiation as a strong promoter of chemical transformations in order to help drive the reactions to completion very quickly during the brief time that they reside in the reaction chamber. This technique is called MACOS, for Microwave-Assisted, Continuous Organic Synthesis and a photograph of a later stage prototype reactor system is shown in Figure 1. So, the group builds all of its own reactor equipment and is strong in reactor design and construction.
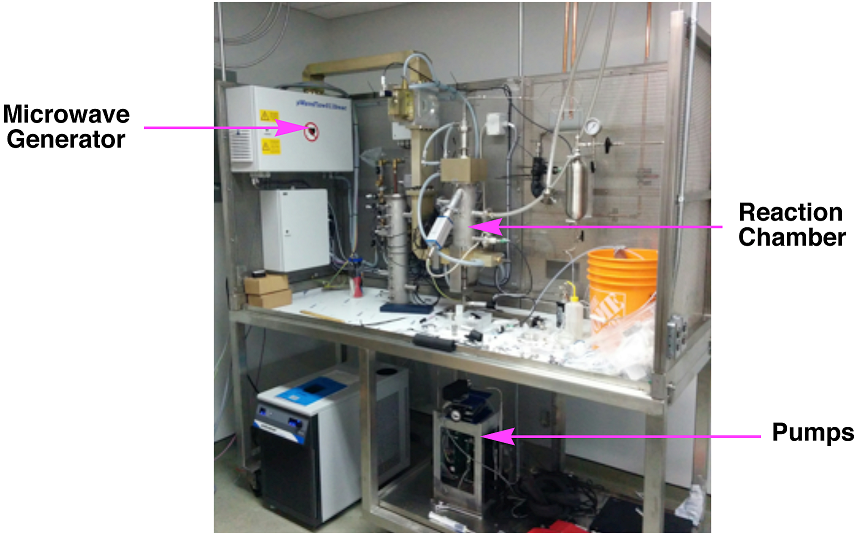
Figure 1. Microwave-Assisted Continuous Organic Synthesis
We have done extensive research using catalysis in flow, both by flowing the catalyst with the reactants1 or by supporting a catalyst inside of the flow reactor (called a packed-bed reactor) and pass the reactants through it. An example of the latter is shown here where we have supported one of our group’s highly reactive Pd ‘PEPPSI’ catalysts that is supported on silica and packed into a bed to enable flowed cross-coupling chemistry to take place.2
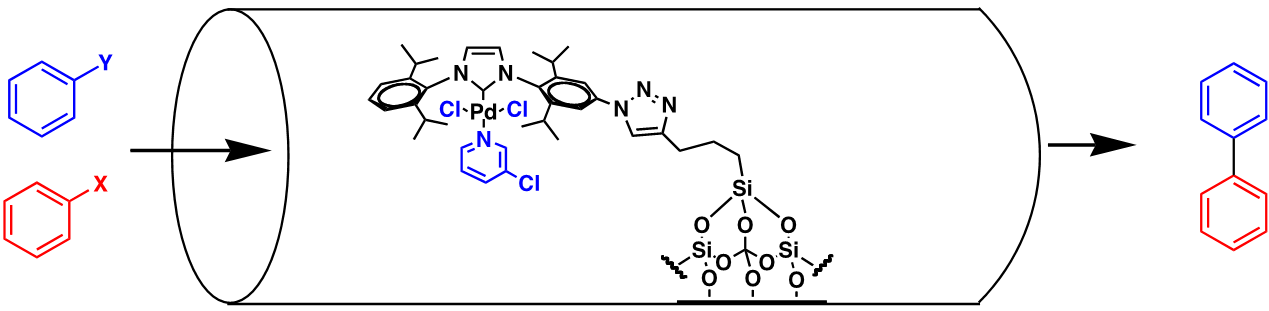
Figure 2. Silica-supported Pd catalyst reactor bed through which cross-coupling reactions are flowed.
We have also invented the use of metal-film-catalyzed organic synthesis using microwave irradiation to promote a wide variety of chemical including films made out of gold, silver, platinum, palladium, and others. A sample Pd-nanoparticle film is depicted in Figure 3.
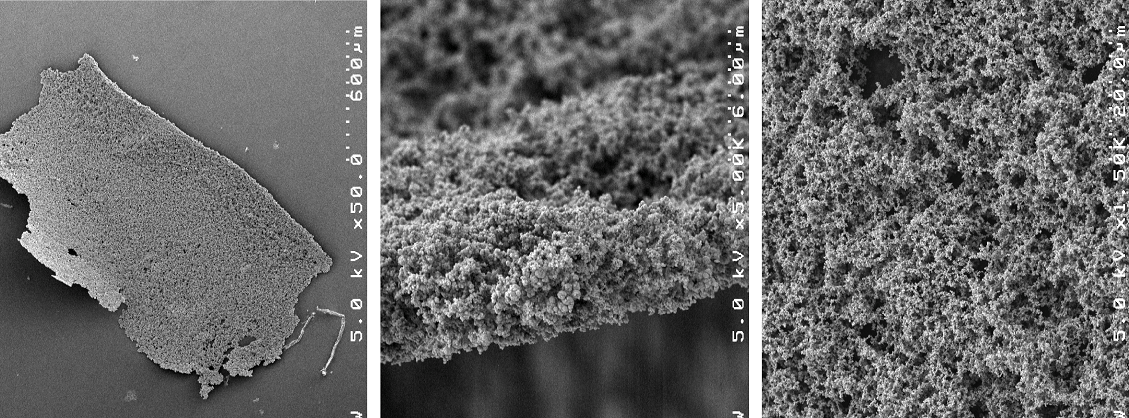
Figure 3. Pd-nanoparticle film that coats reactor wall and catalyses organic reactions.
As only small reactor volumes are present at any point in time, an area that flow chemistry can make a major impact on is the chemistry of high-reactive, but unstable intermediates. Azides and diazonium salts are incredibly versatile reactive intermediates, but are plagued with a track history of explosions due to their incredibly high reactivity. We have made advances in the flow chemistry of both azides4 and diazonium salts (See Scheme 1).5
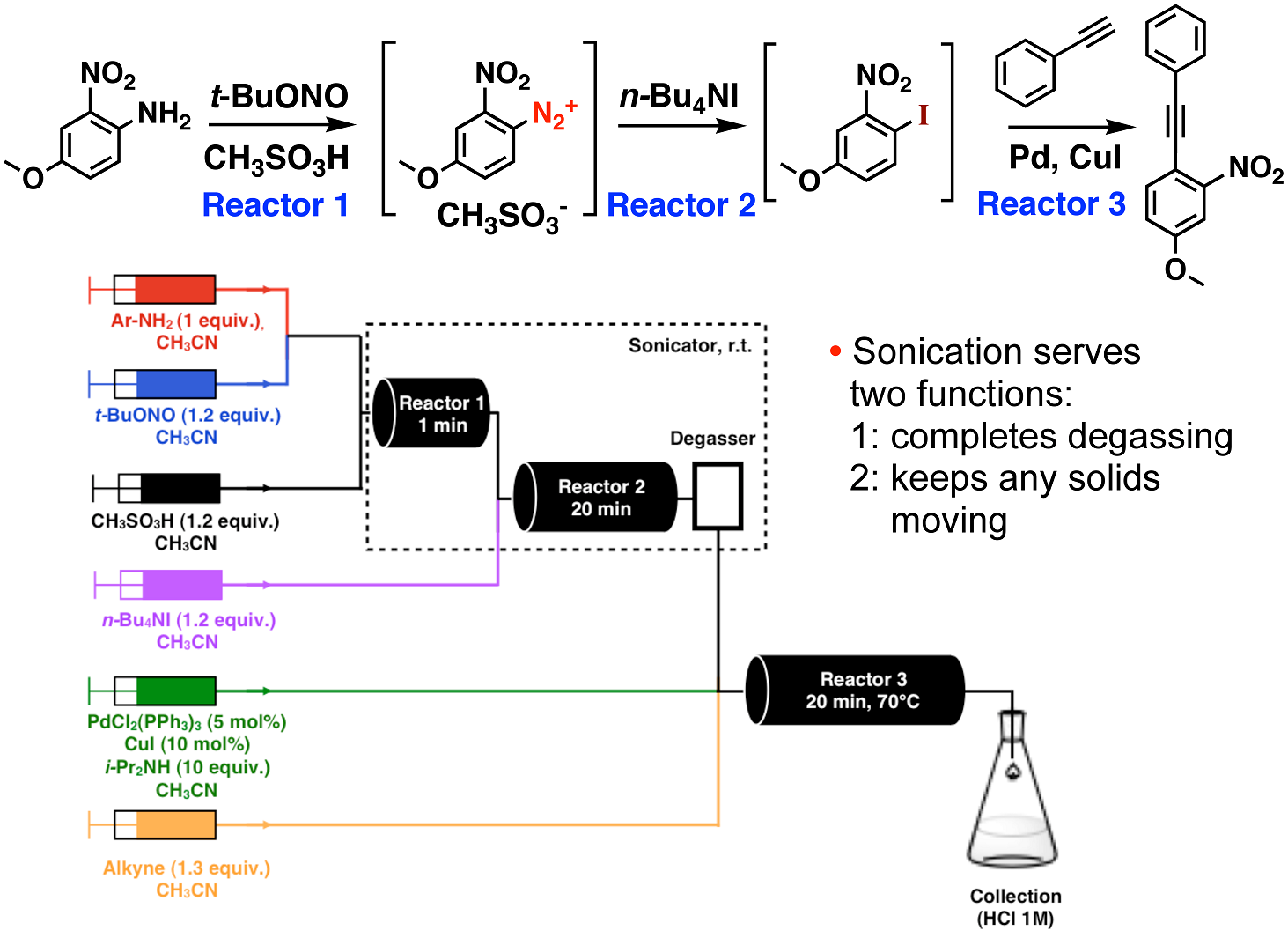
Scheme 1. Three-step diazotization, iodide-capture, Sonogashira cross-coupling.
The broad, multi-disciplinary program area in our group has both synthetic chemists from the Faculty of Science and chemical and computer engineers from the Faculty of Engineering. We collaborate with a large number of companies and our students and post-docs have spent time in the labs of the industrial partner.
Representative Publications (see full list of publication for all manuscripts):
1. Comer, E.; Organ, M. G. J. Am. Chem. Soc. 2005, 127, 8160-8167
2. Price, G. A.; Bogdan, A. R.; Aguirre, A. L.; Iwai, T.; Djuric, S. W.; Organ, M. G. Catalysis Science & Technology 2016, 6, 4733 – 4742
3. Shore, G.; Organ, M. G. Chem. Eur. J. 2008, 14, 9641-9646; Shore, G.; Morin, S.; Organ, M. G. Angew. Chem. Int. Ed. 2006, 45, 2761-2766.
4. Teci, M.; Tilley, M.; McGuire, M. A.; Organ, M. G. Org. Proc. Dev. Res. 2016, 20. ASAP; Painter, T. O.; Thornton, P. D.; Orestano, M.; Santini, C.; Organ, M. G.; Aubé, Chem. Eur. J. 2011, 17, 9595-9598.
5. Teci, M.; Tilley, M.; McGuire, M. A.; Organ, M. G. Chem. Eur. J. 2016, 22. Nalivela, K. S.; Tilley, M.; McGuire, M. A.; Organ, M. G. Chem. Eur. J. 2014, 20, 6603-6607.