Research
Catalysis
Research in the area of catalysis follows two related tracks in our group: 1) mechanistic study and 2) applying catalysis to solve synthetic challenges that otherwise are difficult, if not impossible.
1) Mechanistic studies in catalysis:
While most catalytic reactions have mechanisms drawn out in textbooks, we learn new things about most reactions every day – one thing is for sure, no reaction is a simply as it may seem on the surface. Our group has been interested in the role of additives and how they impact catalytic reactions. In short, while not a starting material in the Negishi reaction, salt additives play a pivotal role that went unnoticed and/or poorly understood for decades. For arylzinc cross-coupling partners, we have proposed that simply table salt is necessary to both adjust the polarity of the cross-coupling solution to breakdown aggregates to make the organozinc more likely to undergo transmetallation. With alkylzincs, we propose (in blue in Scheme 1) that a different role for the salt exists leading to the formation of a more nucleophilic zincate (RZnX3)-2 and that that is the transmallating species and not the simple organometallic (in red) or the lower zincate (RZnX2)-1 as has been proposed by others.1
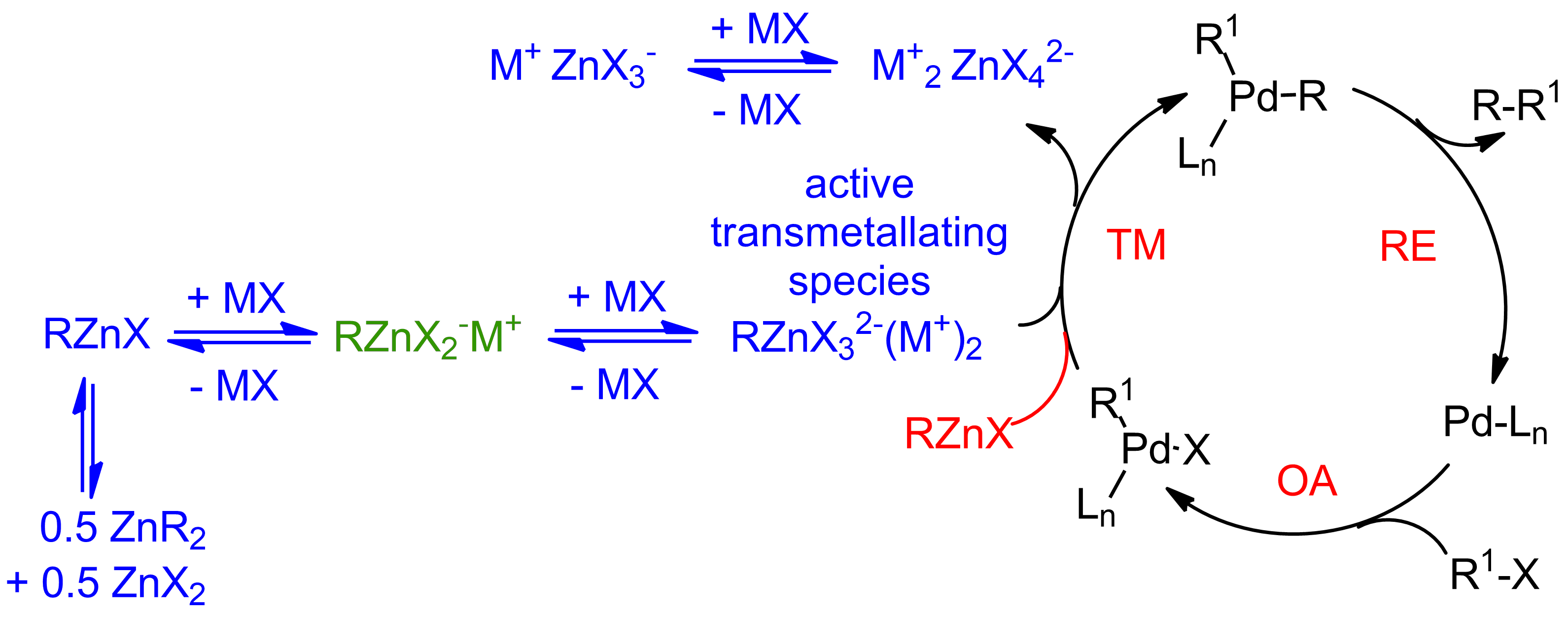
Scheme 1. Our proposed mechanism of the Negishi Reaction
We look at the mechanism of a number of cross coupling reactions (e.g., Suzuki Miyaura, Negishi, and cross coupling with nitrogen and sulfur nucleophiles) using a breadth of tools including kinetics, mass spectrometry, NMR spectroscopy, and calculations (in collaboration).
2) Solving old problems with new catalysts:
The importance of cross-coupling to organic synthesis cannot be overstated as evidenced by the awarding of the 2010 Nobel Prize to Negishi, Suzuki, and Heck. Earlier studies focused primarily on the coupling to two different aryl rings together – itself a major feat at the time and still the best and most general strategy to assemble to aromatic rings together. In addition to the formation of C-C bonds, cross-coupling can now be used essentially to make any linkage (e.g., C-N, C-O, C-S). Once it became clear that transition metals could be used to assemble electrophiles and nucleophiles, the bar has been raised to try to do such couplings with selectivity. For example, primary amines (Scheme 2a)2 or ammonia (Scheme 2b)3 can couple to one aryl electrophile, or it can over couple to generate the diaryl product.
a)
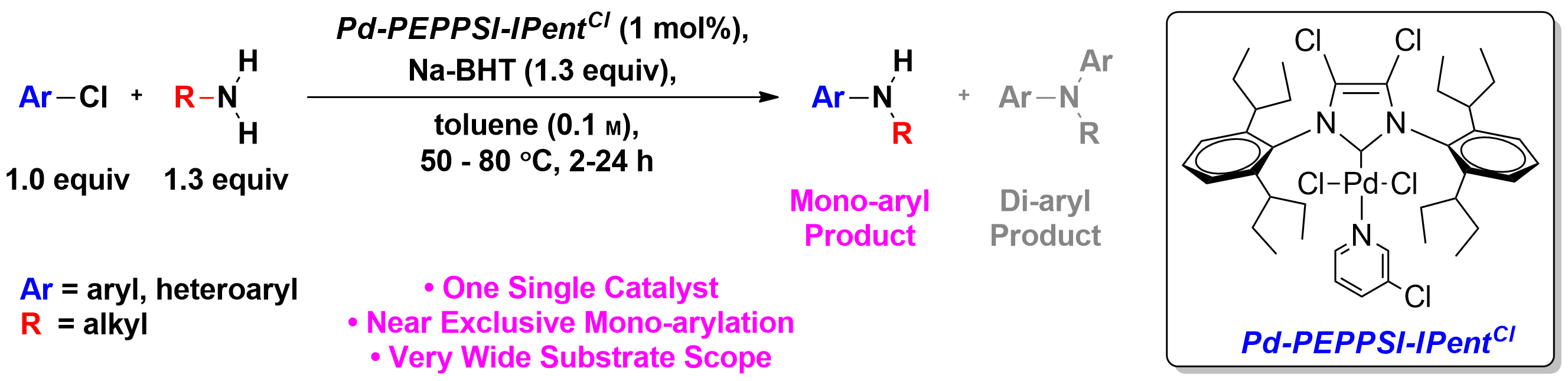
b)
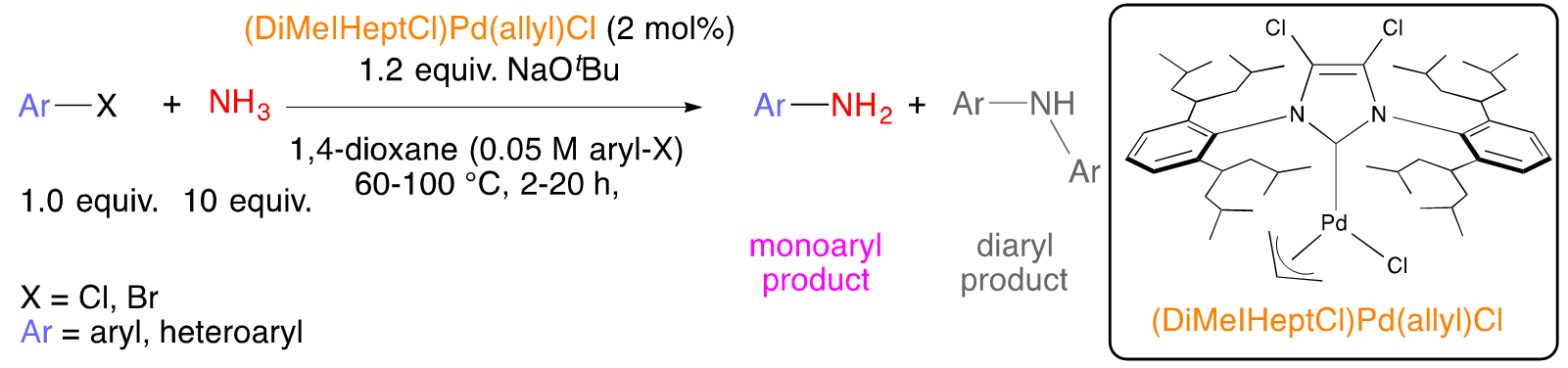
Scheme 2. Selective (hetero)arylation of a) primary amines and b) ammonia
Our group has also been highly active in the cross coupling of secondary nucleophiles to (hetero)arylhalides to produce tertiary centres adjacent to the ring without any migratory insertion that leads to the productions of isomers (Scheme 3).4
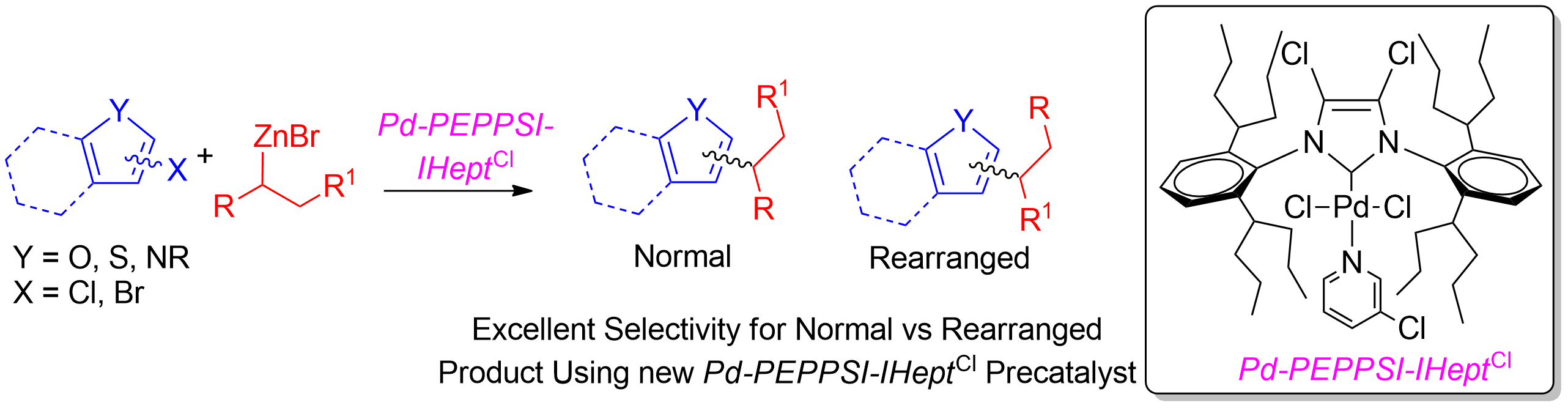
Scheme 3. Selective coupling of secondary alkylzinc nucleophiles to (hetero)aryl halides.
We collaborate with a large number of companies and our students and post-docs have spent time in the labs of the industrial partner.
Representative Publications (see full list of publication for all manuscripts):
1. McCann, L. A.; Organ, M. G. Angew. Chem. Int. Ed. 2014, 53, 4386-4389; McCann, L. C,; Hunter, H. N.; Clyburne, J. A. C.; Organ, M. G. Angew. Chem. Int. Ed. 2012, 51, 7024-7027; Hunter, H. N.; Hadei, N.; Blagojevic, V.; Patschinski, P.; Achonduh, G. T.; Avola, S.; Bohme, D. K.; Organ, M. G. Chem. Eur. J. 2011, 17, 7845-7851.
2. Sharif, S.; Rucker; R. P.; Chandrasoma, N.; Mitchell, D.; Rodriguez, M. J.; Pompeo, M.; Froese, R. D. J.; Organ, M. G. Angew. Chem. Int. Ed. 2015, 54, 9507-9511.
3. Lombardi, C.; Day, J.; Chandrasoma, N.; Mitchell, D.; Rodriguez, M. J.; Farmer, J. L.; Organ, M. G. Organometallics 2016 (Submitted).
4. Atwater, B.; Chandrasoma, N.; Mitchell, D.; Rodriguez, M. J.; Organ, M. G. Chem. Eur. J. 2016, 22, 14531-4; Atwater, B.; Chandrasoma, N.; Mitchell, D.; Rodriguez, M. J.; Pompeo, M.; Froese, R. D. J.; Organ, M. G. Angew. Chem. Int. Ed. 2015, 54, 9502 –9506.
Synthetic Chemistry In Flow
The synthesis of organic molecules has been conducted traditionally in a container such as a round-bottom flask on the lab scale, or in a large, often metal (e.g., stainless steel or hastelloy) stir-tank reactor on the manufacturing scale. This is referred to as ‘batch chemistry’. The production of large-quantity commodity chemicals, such as polymers and ammonia, are prepared in a different format, i.e., by pumping the reaction mixture continuously through a tube – this is called ‘flow chemistry’. Flow chemistry is a very effective way of synthesizing molecules as only small amounts of reactants are in the rube at any one time. This allows for greater control over mass and heat transfer in the reactor that leads to better chemical process, which leads to more efficient and cleaner transformations making flow both a ‘green’ technology and much safer owing to the small amounts of material undergoing reaction at any point in time. We are working at adapting flow chemistry to the preparation of organic molecules with the goal of realizing these same benefits in the preparation of high-value compounds, such as pharmaceutical agents.
Research in the group focuses on effective ways to utilize flow chemistry to realize benefits over the traditional batch processes. The group has pioneered the use of microwave irradiation as a strong promoter of chemical transformations in order to help drive the reactions to completion very quickly during the brief time that they reside in the reaction chamber. This technique is called MACOS, for Microwave-Assisted, Continuous Organic Synthesis and a photograph of a later stage prototype reactor system is shown in Figure 1. So, the group builds all of its own reactor equipment and is strong in reactor design and construction.
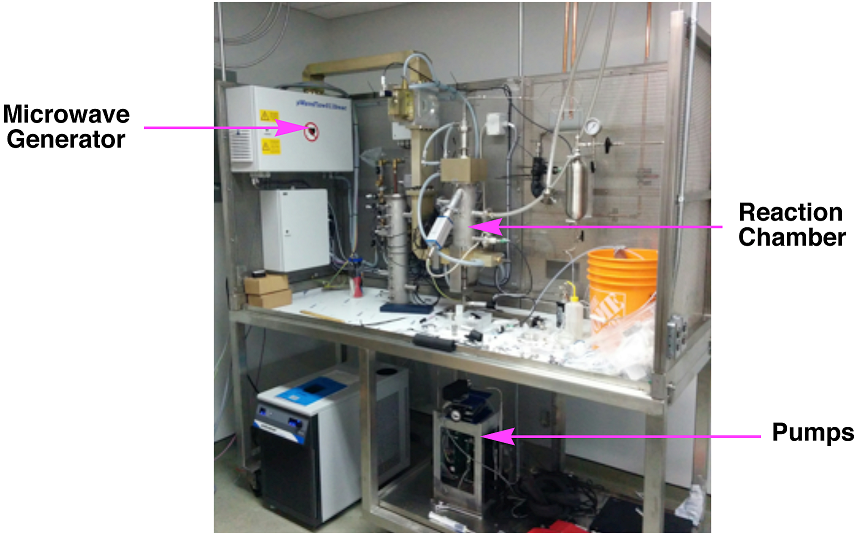
Figure 1. Microwave-Assisted Continuous Organic Synthesis
We have done extensive research using catalysis in flow, both by flowing the catalyst with the reactants1 or by supporting a catalyst inside of the flow reactor (called a packed-bed reactor) and pass the reactants through it. An example of the latter is shown here where we have supported one of our group’s highly reactive Pd ‘PEPPSI’ catalysts that is supported on silica and packed into a bed to enable flowed cross-coupling chemistry to take place.2
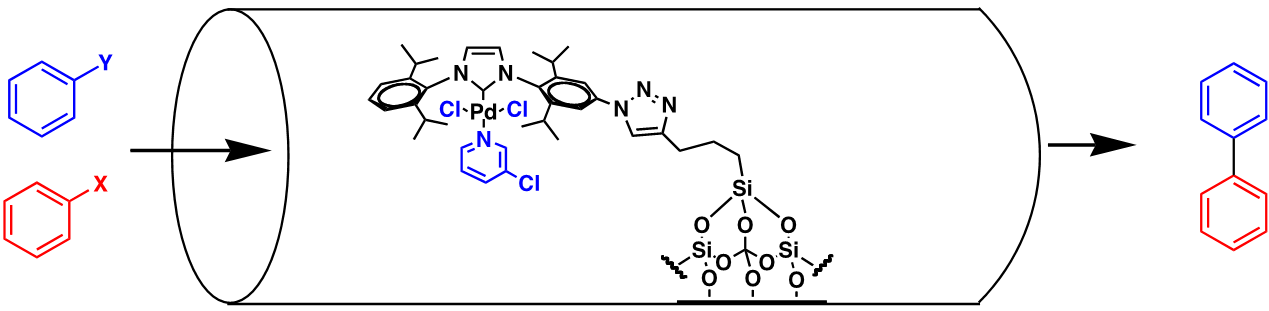
Figure 2. Silica-supported Pd catalyst reactor bed through which cross-coupling reactions are flowed.
We have also invented the use of metal-film-catalyzed organic synthesis using microwave irradiation to promote a wide variety of chemical including films made out of gold, silver, platinum, palladium, and others. A sample Pd-nanoparticle film is depicted in Figure 3.
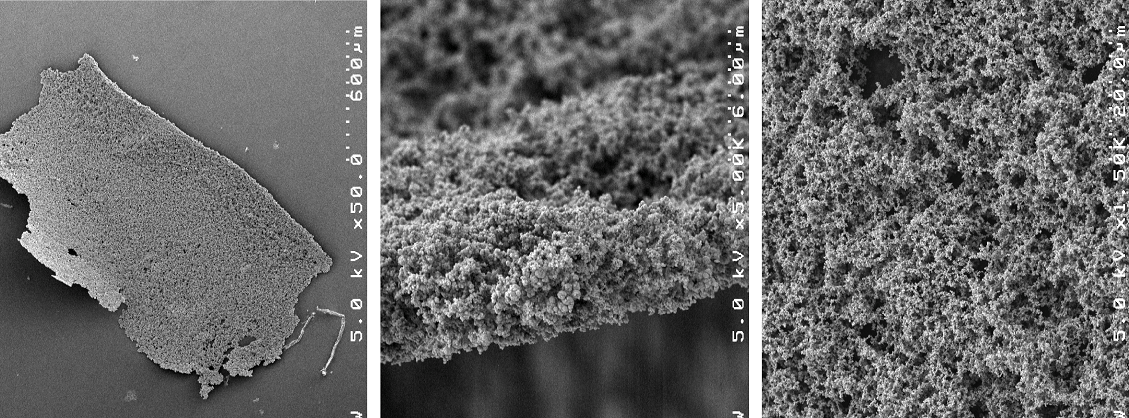
Figure 3. Pd-nanoparticle film that coats reactor wall and catalyses organic reactions.
As only small reactor volumes are present at any point in time, an area that flow chemistry can make a major impact on is the chemistry of high-reactive, but unstable intermediates. Azides and diazonium salts are incredibly versatile reactive intermediates, but are plagued with a track history of explosions due to their incredibly high reactivity. We have made advances in the flow chemistry of both azides4 and diazonium salts (See Scheme 1).5
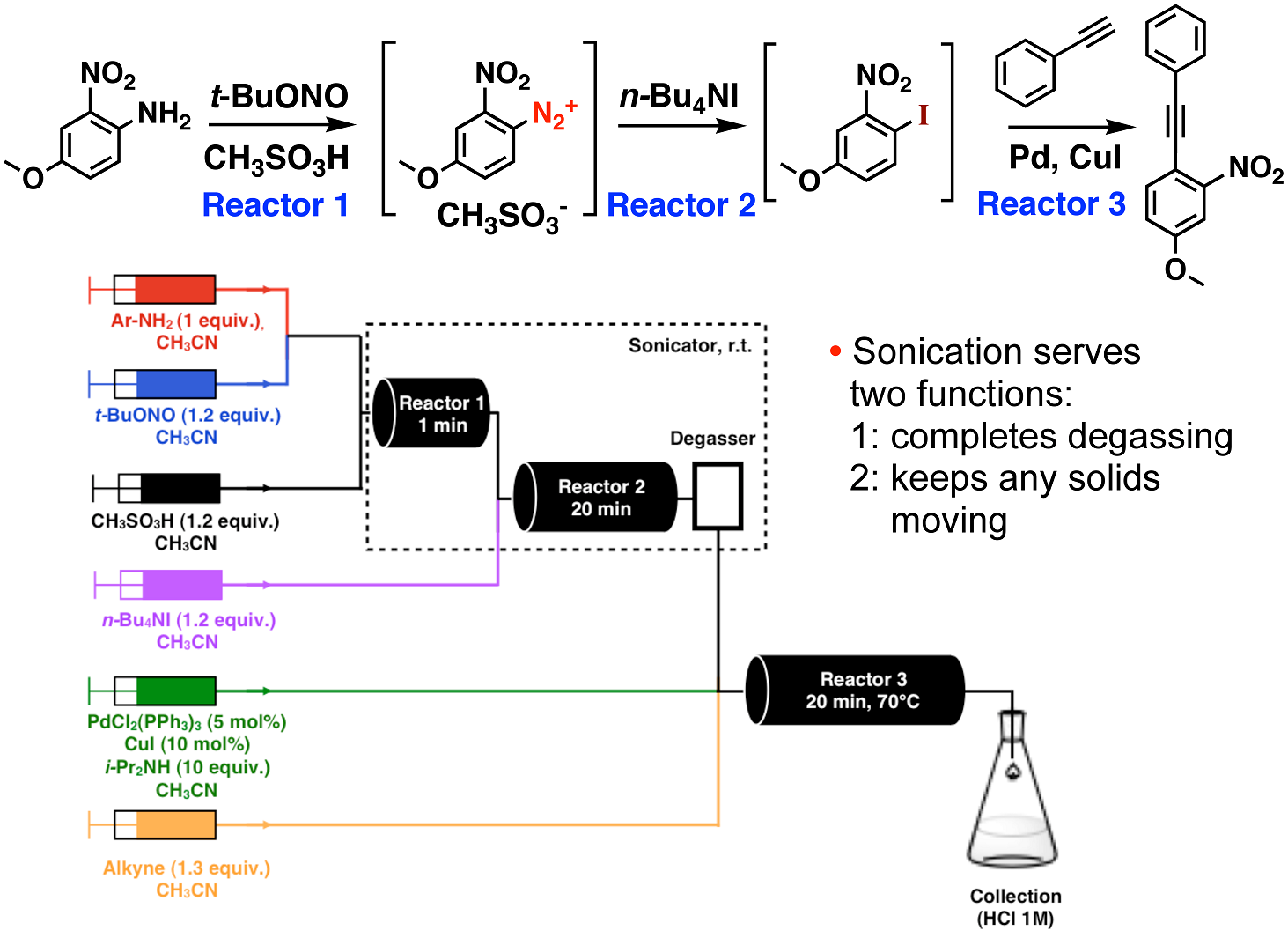
Scheme 1. Three-step diazotization, iodide-capture, Sonogashira cross-coupling.
The broad, multi-disciplinary program area in our group has both synthetic chemists from the Faculty of Science and chemical and computer engineers from the Faculty of Engineering. We collaborate with a large number of companies and our students and post-docs have spent time in the labs of the industrial partner.
Representative Publications (see full list of publication for all manuscripts):
1. Comer, E.; Organ, M. G. J. Am. Chem. Soc. 2005, 127, 8160-8167
2. Price, G. A.; Bogdan, A. R.; Aguirre, A. L.; Iwai, T.; Djuric, S. W.; Organ, M. G. Catalysis Science & Technology 2016, 6, 4733 – 4742
3. Shore, G.; Organ, M. G. Chem. Eur. J. 2008, 14, 9641-9646; Shore, G.; Morin, S.; Organ, M. G. Angew. Chem. Int. Ed. 2006, 45, 2761-2766.
4. Teci, M.; Tilley, M.; McGuire, M. A.; Organ, M. G. Org. Proc. Dev. Res. 2016, 20. ASAP; Painter, T. O.; Thornton, P. D.; Orestano, M.; Santini, C.; Organ, M. G.; Aubé, Chem. Eur. J. 2011, 17, 9595-9598.
5. Teci, M.; Tilley, M.; McGuire, M. A.; Organ, M. G. Chem. Eur. J. 2016, 22. Nalivela, K. S.; Tilley, M.; McGuire, M. A.; Organ, M. G. Chem. Eur. J. 2014, 20, 6603-6607.
Medicinal Chemistry
Research in this area is targeted at the discovery of molecules to be used as therapeutics in the treatment of infectious disease in collaboration with Professor Eric Brown’s group in the Department of Biochemistry and the Michael G. Degroote Institute for Infectious Disease Research at McMaster University.
“Drug combinations are valuable tools for studying biological systems. Although much attention has been given to synergistic interactions in revealing connections between cellular processes, antagonistic interactions can also have tremendous value in elucidating genetic networks and mechanisms of drug action.” The Brown group has exploited the power of antagonism in a high-throughput screen for molecules that suppress the activity of targocil, an inhibitor of the wall teichoic acid (WTA) flippase in Staphylococcus aureus. “Well-characterized antagonism within the WTA biosynthetic pathway indicated that early steps would be sensitive to this screen; however, broader interactions with cell wall biogenesis components suggested that it might capture additional targets. A chemical screening effort using this approach identified clomiphene, a widely used fertility drug, as one such compound. Mechanistic characterization revealed the target was the undecaprenyl diphosphate synthase, an enzyme that catalyzes the synthesis of a polyisoprenoid essential for both peptidoglycan and WTA synthesis. The work sheds light on mechanisms contributing to the observed suppressive interactions of clomiphene and in turn reveals aspects of the biology that underlie cell wall synthesis in S. aureus. Further, this effort highlights the utility of antagonistic interactions both in high-throughput screening and in compound mode of action studies. Importantly, clomiphene represents a lead for antibacterial drug discovery.”1
Professor Brown’s group has uncovered three molecules that effectively inhibit undecaprenyl diphosphate synthase, Chlomiphene, Tarocil, and Ticlopidine (Figure 1).
Figure 1. Structures of the three lead candidates, Chlomiphene, Tarocil, and Ticlopidine, which are inhibitors of undecaprenyl diphosphate synthase.
The crystal structures of the target enzyme with Chlomiphene bound is shown in Figure 2.
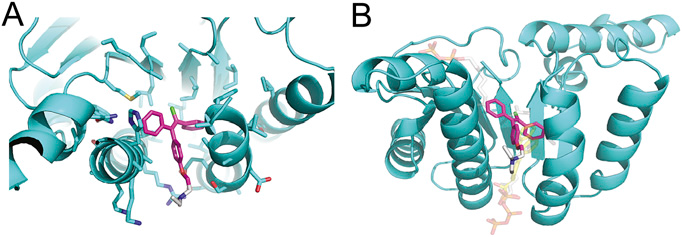
Figure 2. Crystal structure of clomiphene bound to E. coli UppS (PDB ID 5CQJ).
(A) Clomiphene (magenta) binds in the hydrophobic channel linking binding sites 1 (above image) and 4 (below image). The gray colored region in the ligand indicates atoms unresolved in the electron density and omitted from refinement. (B) Comparison of clomiphene binding position with substrate and inhibitor. Clomiphene (magenta) binds in the hydrophobic channel linking binding sites 1 and 4. The binding position overlaps with the binding of FPP in sites 1 and 4 (substrate, white, PDB ID code 1V7U) and high affinity bisphosphonate inhibitor in site 4 (yellow, PDB ID code 2E98). (Figure is taken from www.pnas.org/cgi/doi/10.1073/pnas.1511751112)
Together with Professor Brown’s team, we are preparing analogues of Chlomiphene, Tarocil, and Ticlopidine to improve their efficacy, while at the same time improve their adsorption, distribution, metabolism, and excretion (ADME) properties in the body.
Representative Publications:
1. Farha, M. A.; Czarny, T. L.; Myers, C. L.; Worrall, L. J.; French, S.; Conrady, D. G.; Wang, Y.; Oldfield, E.; Strynadka, N. C. J.; Brown, E. D. PNAS 2015, 11048–11053.