Medicinal Chemistry
Research in this area is targeted at the discovery of molecules to be used as therapeutics in the treatment of infectious disease in collaboration with Professor Eric Brown’s group in the Department of Biochemistry and the Michael G. Degroote Institute for Infectious Disease Research at McMaster University.
“Drug combinations are valuable tools for studying biological systems. Although much attention has been given to synergistic interactions in revealing connections between cellular processes, antagonistic interactions can also have tremendous value in elucidating genetic networks and mechanisms of drug action.” The Brown group has exploited the power of antagonism in a high-throughput screen for molecules that suppress the activity of targocil, an inhibitor of the wall teichoic acid (WTA) flippase in Staphylococcus aureus. “Well-characterized antagonism within the WTA biosynthetic pathway indicated that early steps would be sensitive to this screen; however, broader interactions with cell wall biogenesis components suggested that it might capture additional targets. A chemical screening effort using this approach identified clomiphene, a widely used fertility drug, as one such compound. Mechanistic characterization revealed the target was the undecaprenyl diphosphate synthase, an enzyme that catalyzes the synthesis of a polyisoprenoid essential for both peptidoglycan and WTA synthesis. The work sheds light on mechanisms contributing to the observed suppressive interactions of clomiphene and in turn reveals aspects of the biology that underlie cell wall synthesis in S. aureus. Further, this effort highlights the utility of antagonistic interactions both in high-throughput screening and in compound mode of action studies. Importantly, clomiphene represents a lead for antibacterial drug discovery.”1
Professor Brown’s group has uncovered three molecules that effectively inhibit undecaprenyl diphosphate synthase, Chlomiphene, Tarocil, and Ticlopidine (Figure 1).
Figure 1. Structures of the three lead candidates, Chlomiphene, Tarocil, and Ticlopidine, which are inhibitors of undecaprenyl diphosphate synthase.
The crystal structures of the target enzyme with Chlomiphene bound is shown in Figure 2.
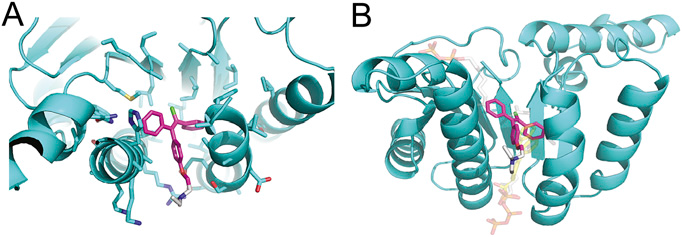
Figure 2. Crystal structure of clomiphene bound to E. coli UppS (PDB ID 5CQJ).
(A) Clomiphene (magenta) binds in the hydrophobic channel linking binding sites 1 (above image) and 4 (below image). The gray colored region in the ligand indicates atoms unresolved in the electron density and omitted from refinement. (B) Comparison of clomiphene binding position with substrate and inhibitor. Clomiphene (magenta) binds in the hydrophobic channel linking binding sites 1 and 4. The binding position overlaps with the binding of FPP in sites 1 and 4 (substrate, white, PDB ID code 1V7U) and high affinity bisphosphonate inhibitor in site 4 (yellow, PDB ID code 2E98). (Figure is taken from www.pnas.org/cgi/doi/10.1073/pnas.1511751112)
Together with Professor Brown’s team, we are preparing analogues of Chlomiphene, Tarocil, and Ticlopidine to improve their efficacy, while at the same time improve their adsorption, distribution, metabolism, and excretion (ADME) properties in the body.
Representative Publications:
1. Farha, M. A.; Czarny, T. L.; Myers, C. L.; Worrall, L. J.; French, S.; Conrady, D. G.; Wang, Y.; Oldfield, E.; Strynadka, N. C. J.; Brown, E. D. PNAS 2015, 11048–11053.