


Our group is interested in asymmetric catalysis by organic molecules. We have developed a new class of hydrazide-based catalyst that catalyzes Diels-Alder cyclizations in aqueous media.
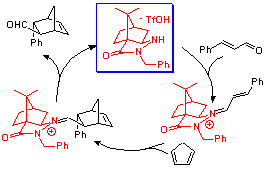
These compounds catalyze a variety of asymmetric transformations with high yields. We are working to expand and explore the chemistry of these novel and highly effective organocatalysts.
The stereodefined synthesis of tetrasubstituted olefins
The synthesis of tetrasubstituted olefins is particularly challenging and methods to form stereodefined, acyclic tetrasubstituted olefins bearing all-different carbon substituents are exceedingly rare. When all-different carbon-linked tetrasubstituted olefins can be prepared, mixtures and low yields are frequently obtained. We have developed an efficient, highly flexible method for the formation of single isomer olefin moieties. E-b-chloro-a-iodo-a,b-unsaturated esters are generated as single isomers in excellent yields from 2-alkynyl esters using a simple, reliable procedure. Sequential coupling reactions provide the desired, sterically congested tetrasubstituted olefins as single isomers in good yields.
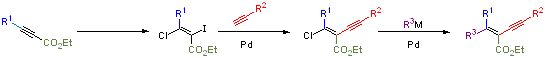
This methodology is currently being explored and expanded, together with applications in total synthesis.
Contact William W. Ogilvie (613) 562-5800 extension 6071 Fax : (613) 562-5170 wogilvie@uottawa.ca
![]()
