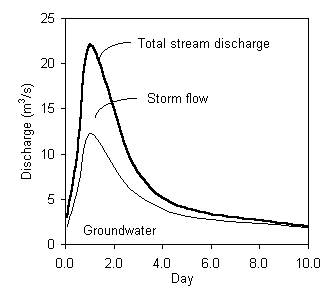
Using the single component separation (d 18O), according to the formulation given earlier in this chapter, the separated hydrograph is:
|
|
|
|
|
|||
|
|
|
|
|
|||
|
|
|
|
|
|||
|
|
|
|
|
|||
|
|
|
|
|
|||
|
|
|
|
|
|||
|
|
|
|
|
|||
|
|
|
|
|
|||
|
|
|
|
||||
|
|
|
|
||||
|
|
|
|
||||
Using the single component separation (d 18O), according to the formulation given earlier in this chapter, the separated hydrograph is:

|
Day
|
Qt
|
Qgw
|
|
|
0.1
|
3
|
2.0
|
|
|
0.5
|
10
|
6.0
|
|
|
1
|
22
|
12.3
|
|
|
2
|
15
|
8.6
|
|
|
3
|
8
|
5.4
|
|
|
5
|
4
|
3.1
|
|
|
10
|
2
|
1.9
|
|
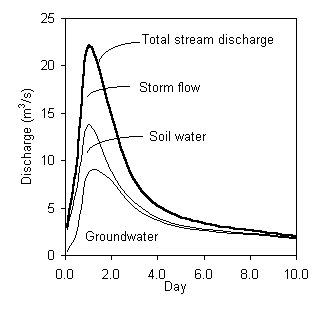
Now using both Si and d18O, the hydrograph can be separated into three discharge components. These are shown in this graph:
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||
|
|
|
|
|
|
|
|||||
|
|
|
|
|
|
|
|||||
|
|
|
|
|
|
|
|||||
|
|
|
|
|
|
|
|||||
|
|
|
|
|
|
|
|||||
|
|
|
|
|
|
|
|||||
|
|
|
|
|
|
|
|||||
|
|
|
|
|
|
|
|||||
|
|
|
|
|
|
|
|||||
|
|
|
|
|
|
|
|||||
|
|
|
|
|
|
|
|||||
|
|
|
|
|
|
|
|||||
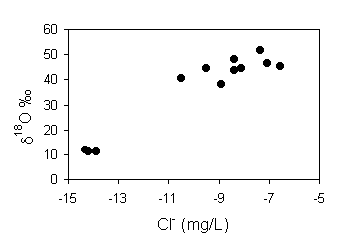
The Cl– content in recharge water is 8 mg/L. The fraction
recharge R is determined from the relationship:
The seasonality of transpiration water loss is evident from a plot of d18O vs. Cl–. There is a reasonably good correlation between these parameters, showing that the greatest enrichments in Cl– occur when precipitation is the most isotopically enriched, i.e. during the summer months when transpiration by crops and other vegetation is at a maximum and recharge at a minimum.
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
770
|
–0.36
|
3.0
|
|
12470
|
0.44
|
3.5
|
|
484
|
–2.64
|
–5.3
|
|
|
495
|
0.01
|
4.6
|
|
574
|
–0.01
|
5.8
|
|
835
|
–3.28
|
–14.1
|
|
|
538
|
0.10
|
5.2
|
|
363
|
–7.60
|
2.6
|
|
1607
|
–3.18
|
–13.5
|
|
|
460
|
–0.31
|
5.1
|
|
363
|
–0.69
|
2.4
|
|
417
|
–3.14
|
–8.0
|
|
|
2450
|
–0.76
|
0.2
|
|
9620
|
0.07
|
1.9
|
|
468
|
–3.26
|
–11.7
|
|
|
11150
|
0.46
|
3.7
|
|
930
|
–1.66
|
–4.3
|
|
1365
|
–3.67
|
–16.6
|
|
|
4170
|
–0.55
|
1.2
|
|
406
|
–1.66
|
–8.0
|
|
546
|
–2.68
|
–8.1
|
|
|
335
|
–0.44
|
4.4
|
|
406
|
–1.42
|
–9.0
|
|
339
|
–1.91
|
–3.4
|
|
|
335
|
–0.40
|
4.0
|
|
380
|
–1.30
|
–4.5
|
|
252
|
–2.89
|
–7.1
|
|
|
6130
|
–0.20
|
2.8
|
|
380
|
–1.19
|
–4.1
|
|
259
|
–2.39
|
–5.8
|
|
|
337
|
–0.51
|
1.2
|
|
382
|
–1.00
|
–1.0
|
|
343
|
–2.42
|
–4.8
|
|
|
337
|
–0.46
|
1.1
|
|
382
|
–0.91
|
–0.9
|
|
304
|
–2.32
|
–2.7
|
|
|
404
|
–0.59
|
1.5
|
|
528
|
–1.45
|
–6.0
|
|
555
|
–3.80
|
–14.3
|
|
|
404
|
–0.54
|
1.4
|
|
644
|
–1.29
|
–4.1
|
|
343
|
–2.70
|
–7.6
|
|
|
577
|
–0.07
|
3.5
|
|
3620
|
–0.68
|
0.1
|
|
555
|
–2.22
|
–4.7
|
|
|
18540
|
1.01
|
5.1
|
|
14760
|
0.84
|
4.1
|
|
468
|
–3.41
|
–10.6
|
|
|
7840
|
–0.06
|
2.6
|
|
740
|
–0.70
|
–0.5
|
|
410
|
–3.45
|
–11.7
|
|
|
437
|
–0.68
|
0.8
|
|
620
|
–0.95
|
–3.3
|
|
1170
|
–3.36
|
–11.4
|
|
|
14050
|
0.70
|
4.4
|
|
11660
|
0.26
|
4.4
|
|
794
|
–2.62
|
–8.1
|
|
|
554
|
–0.11
|
3.0
|
|
5480
|
–0.26
|
1.3
|
|
302
|
–2.73
|
–5.7
|
|
|
374
|
–1.10
|
0.6
|
|
19680
|
1.26
|
6.1
|
|
306
|
–3.44
|
–14.0
|
|
|
374
|
–1.00
|
0.5
|
|
357
|
–3.76
|
–16.2
|
||||
|
|
9000
|
0.17
|
3.4
|
|
257
|
–3.66
|
–12.4
|
|
264
|
–3.52
|
–14.7
|
|
|
4950
|
–0.68
|
2.2
|
|
293
|
–2.89
|
–9.5
|
|
293
|
–3.04
|
–11.6
|
|
|
650
|
–1.27
|
–2.8
|
|
295
|
–2.13
|
–4.3
|
|
519
|
–2.90
|
–12.7
|
|
|
523
|
–0.77
|
3.2
|
|
278
|
–3.09
|
–9.7
|
|
335
|
–2.26
|
–5.7
|

= 14.2 (1 – 0.4) = 8.52
= 8.52 + 9.1 = 17.6‰
= –0.83 – 17.6 · ln (0.023)
= 64.1‰
The percent mixing would be determined using either Cl– or
d18O, from the ratio:
e.g. AL42 = 100 ´ (9620 – 484)/(19,500 – 484) = 48%
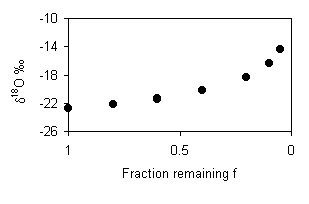
This evaporative loss can be calculated using the same Rayleigh formulation
given above. However, in this calculation, the final d18O
is the mean of the low salinity samples, and the initial d18O
value is found by extrapolating horizontally (constant d2H)
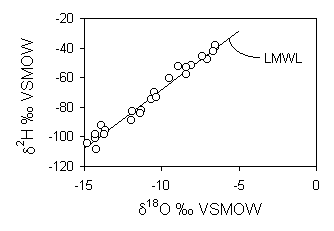
to the LMWL. This can also be calculated by using the corresponding d2H
value for the average of these data (0.03‰) in the equation for the LMWL:
d18O = –1.8‰
–0.83 = –1.8 – 17.6 · ln f
f = 0.95
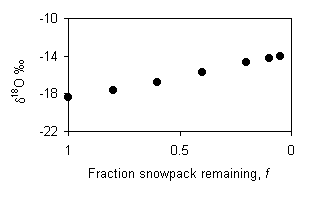
If the melting takes place due to rain, then the runoff will be a mixture of the rain and snowmelt that has re-equilibrated with the remaining snowpack. The d18O value of the runoff will depend on the amount of rain, and the amount of melting, but will initially be less than –16.8‰ (d18Orain + e18Ow-i = –14 – 2.8 = –16.8‰) and will evolve towards the isotopic composition of the rain. This diagram assumes equal contributions from the rain and snowmelt at the outset, evolves toward 100% rain.
|
|
|
||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||
|
|
|
|
|||
|
|
|
|
|||
|
|
|
|
|||
|
|
|
|
|||
|
|
|
|
|||
|
|
|
|
|||
|
|
|
|
|||
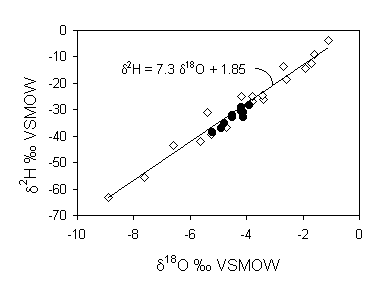
The increase in salinity is due to transpiration by crops. This is a non-fractionating process and so does not affect the isotopic composition of the groundwater. Improving irrigation methods will not cut down on water loss. The average water loss is reflected by the increase in salinity. The average Cl– content in the precipitation is 9.4 mg/L, whereas in the groundwater it is 134 mg/L. This is a 14.2 fold increase in salinity and so the residual water fraction f = 1/14.2 = 0.07. This means that 93% of the water has been lost to transpiration.