Novel antifreeze glycoproteins
Our work with the antifreeze glycoproteins centers on the design and synthesis of various structural and/or functional analogues which are used as biological probes to study how these compounds interact with ice.
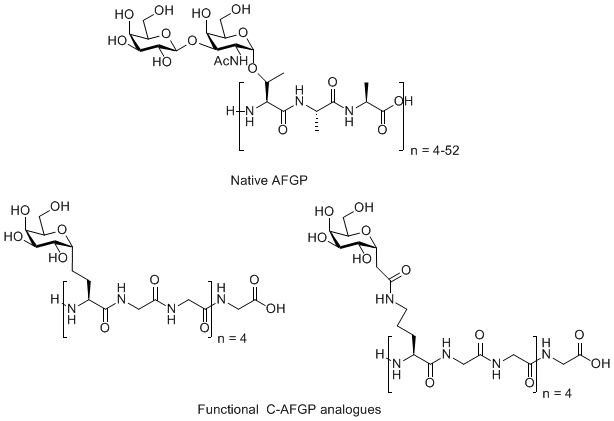
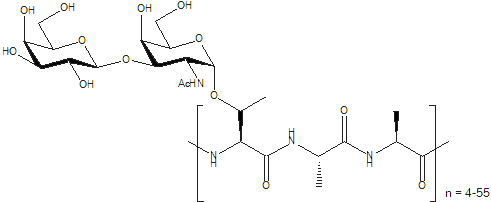
Antifreeze Glycoproteins (AFGPs) have the ability to inhibit the growth of ice crystals in vivo. These compounds are essential to the survival of organisms that inhabit subzero environments such as Arctic and Antarctic teleost fish. The structure of a typical AFGP is shown below.
Figure 1. A typical antifreeze glycoprotein (AFGP)
Since the ability to inhibit the growth of ice crystals is very attractive from a practical perspective, these compounds (and structurally related analogues) have potential medical, industrial and commercial applications. Unfortunately, the major source of native AFGP is Teleost fish and the isolation and purification of these compounds is a labor-intensive and costly process.
a) C-Linked AFGP Analogues: Structural and Functional Mimetics of AFGPs
The rational design and synthesis of novel synthetic antifreezes with enhanced stability and activity is an attractive alternative to the isolation and purification of native AFGP. The design of such compounds will be closely based upon the structure of native AFGPs. While the mechanism by which AFGPs inhibit ice crystal growth is well understood, the nature of the ice-protein and/or ice-carbohydrate interactions remains a source of debate. Similarly, an explanation for the observed ice-binding specificity and affinity has failed to emerge.
In an effort to further elucidate the mechanism by which these glycoproteins function, our laboratory is conducting detailed structure-function studies using various synthetic carbon-linked (C-linked) AFGP analogues. Our synthetic strategy is centered on the preparation of structurally diverse building blocks that are assembled into AFGP mimics using conventional solid phase synthesis. A model illustrating this approach is shown below.
Figure 2. Model for AFGP structure-activity relationship studies
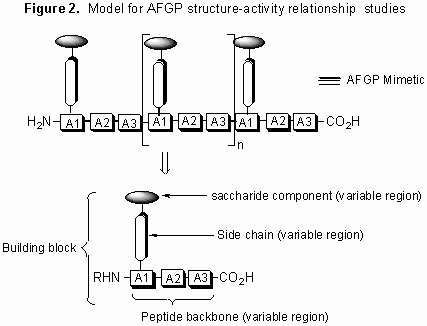
This synthesis is general in nature and thus allows for the systematic variation of saccharide structure, amino acid side chain and polypeptide backbone.
b) Custom-tailored Antifreeze Activity:
In addition to the design of AFGP analogues with enhanced chemical and biological stability, we also seek to design antifreeze glycoconjugates with custom-tailored antifreeze protein-specific activity. Biological antifreezes possess primarily two types of activity. The first is thermal hysteresis which is a depression of freezing point relative to melting point. In conjunction with this, "dynamic ice shaping" is also observed (Figure 3).
Figure 3. Single ice crystals grown in distilled water (A) and a solution of AFGP 8 (B, 10 mg/mL of AFGP8)

In addition to thermal hysteresis, biological antifreezes also have the ability to inhibit recrystallization in frozen samples (Figure 4). While recrystallization-inhibition activity is a very desirable property that makes these compounds attractive as cryoprotectants for cells, tissues and organs, the dynamic ice shaping associated with thermal hysteresis causes significant cellular damage at temperatures outside of the TH gap. Consequently, native antifreeze glycoprotein is a poor cryoprotectant. During the last several years, our laboratory has designed several C-linked AFGP analogues (Figure 5) that are extremely potent recrystallization inhibitors but do not posses thermal hysteresis. As a result, these compounds are ideally suited for many cryomedical applications. Our laboratory is presently exploring such applications in various biological systems.
Figure 4. Inhibition of Recrystallization in ice (both images have the same magnification).
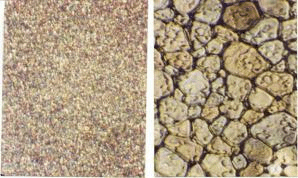
AFGP 8 (10 mg/mL) PBS control (No inhibition)
Figure 5. Native antifreeze glycoprotein (AFGP) and C-linked AFGP analogues.